A Route Toward the Island of Stability
Scientists have synthesized an isotope of the superheavy element livermorium using a novel fusion reaction. The result paves the way for the discovery of new chemical elements.
How and where in the Universe are the chemical elements created? How can we explain their relative abundance? What is the maximum number of protons and neutrons that the nuclear force can bind in a single nucleus? Nuclear physicists and chemists expect to find answers to such questions by creating and studying new elements. But as elements get more and more massive, they become harder and harder to synthesize. The heaviest elements discovered so far were created by bombarding high-atomic-number (high-Z) actinide targets with beams of calcium-48 (48Ca). This isotope is particularly suited to such experiments because of its peculiar nuclear configuration, in which the number of neutrons and protons are both “magic numbers.” Yet this approach could not produce elements beyond oganesson (proton number, Z = 118). Now a team at Lawrence Berkeley National Laboratory (LBNL), California, has synthesized a superheavy element, livermorium-290 (Z = 116), using a beam of titanium-50, which isn’t doubly magic [1]. By removing the requirement of doubly magic nuclei, the work opens up new paths to producing elements beyond element 118, physics.aps.org.
The heaviest naturally abundant element on Earth and in the Solar System is uranium, with an atomic number of 92. Stars are the main natural production sites of the naturally occurring elements. During its lifetime, a star can create atomic nuclei up to iron (Z = 26) in fusion reactions. Nuclei beyond iron are produced at the end of a star’s life, in supernovae or neutron-star mergers—violent events that generate huge densities of free neutrons [2]. These neutrons are captured by iron “seed nuclei,” and ensuing beta decays turn some of the neutrons into protons, thereby raising the atomic number and creating the elements up to uranium (Z = 92). Presumably, this neutron-capture process can lead to much heavier nuclei, entering deep into the territory of superheavy elements with atomic numbers far beyond 100. The periodic table currently contains 26 elements beyond uranium that have been artificially created. However, we do not know how many of them can also arise naturally.
All the superheavy elements known today were synthesized by facilitating fusion reactions between light projectile nuclei and heavy target nuclei [3]. The creation of a superheavy element is a rare event. To observe one nucleus of element 118, for example, the target foil must be bombarded with a billion billion projectiles. In a typical experiment, this takes about a fortnight. Producing a single gram of this element would require more than 1019 years, corresponding to a billion times the age of our Universe. And yet, at the end of this production process, you would have nothing to show for it. This is because superheavy elements are fleeting: All of them are radioactive with short half-lives, some as short as a millionth of a second—just long enough to travel from their point of origin to the detectors, but too short to form even microscopic pieces of matter. This gives an idea of the challenges faced by researchers in this field.
Experiments have shown that the yield of superheavy fusion products is largest when either the projectile or the target nucleus is what’s called a doubly magic nucleus—a nucleus with fully occupied proton and neutron shells. This property enhances not only their stability but the stability of the fusion products too. All elements with atomic numbers 107 and greater were discovered by colliding such nuclei. Elements 107 to 113 were discovered by experiments in which the doubly magic lead isotope 208Pb (82 protons, 126 neutrons) was used as the target material [4, 5], while elements 114 to 118 were discovered in fusion reactions between actinide targets and the doubly magic calcium isotope 48Ca (20 protons, 28 neutrons) [6].
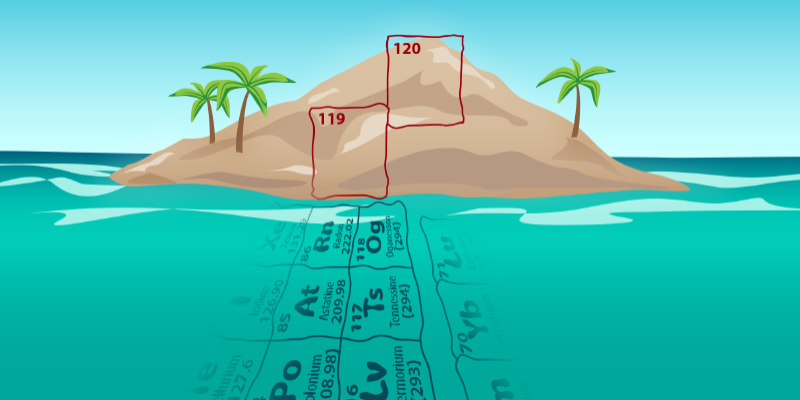
A major goal of today’s superheavy element research is the synthesis of elements 119 and 120. Element 120 is of particular interest because some theoretical models predict a region of enhanced stability for nuclei at and around this proton number, the so-called island of stability [7]. This enhanced stability does not mean that these nuclei are nonradioactive. They are, but their half-lives are expected to be much longer than those of other superheavy isotopes. If this island of stability does indeed exist, it might be reached via the neutron-capture process that occurs in dying stars.
The production yields for elements 119 and 120 are likely to be tiny. Researchers expect that an experiment will have to run for half a year or longer to observe a single nucleus. But that’s not the only difficulty. In trying to synthesize these elements, we face an additional challenge: The 48Ca projectiles favored by researchers are no longer applicable because there is no suitable target material for them to combine with. To reach Z values of 119 and 120 using a 48Ca beam would require targets of einsteinium (Z = 99) and fermium (Z = 100), respectively. These unstable nuclei have half-lives shorter than the length of the experiment, and they are only available in microgram quantities—far less than the 10 or so milligrams required. Instead, we have to enter unknown territory and use nonmagic projectile and target nuclei. Such a “leap into the unknown” has been made in the past decade by researchers in other labs (see Reference [8] and the references therein), but clear evidence of the sought-after elements 119 and 120 did not show up.
The team at LBNL adopted a systematic, step-by-step approach, aiming not for elements beyond 118 but for isotopes of an already known superheavy element: livermorium (Z = 116, with four known isotopes). The third-heaviest element in the current periodic table, livermorium, was discovered in 2004 by a Russian–American collaboration at the Flerov Laboratory of Nuclear Reactions (FLNR) in Russia in studies that used 48Ca projectiles and curium targets [9].
The LBNL researchers sought to create livermorium using an untested combination. They bombarded plutonium targets with 50Ti projectiles—both nonmagic nuclei—and observed two nuclei of the livermorium isotope 290Lv. This marks the first time that one of the heaviest known elements has been synthesized with a nonmagic collision system, making the experiment an important proof of principle. The result demonstrates that fusion reactions of nonmagic nuclei have the potential to produce isotopes of the heaviest known elements and, one can expect, of new elements.
As well as enabling the discovery of new elements, reactions with nonmagic projectiles offer the chance to discover many new isotopes of known elements with atomic numbers ranging from 104 to 118. About 110 different superheavy isotopes are known to date. About 50 further isotopes are expected to exist but are not reachable by conventional fusion reactions using 208Pb targets or 48Ca beams. Reactions with nonmagic systems would allow this gap to be filled. It is worth noting that the FLNR has also announced results on the production of element 116 through collisions involving a non-doubly-magic nucleus heavier than 48Ca [10]. Using fusion reactions of 54Cr and 238U, the FLNR claims the discovery of a new isotope of element 116 (288Lv), but the result has yet to appear in a peer-reviewed publication.
References
J. M. Gates et al., “Toward the discovery of new elements: Production of livermorium (Z = 116) with 50Ti,” Phys. Rev. Lett. 133, 172502 (2024).
K. Langanke and F.-K. Thielemann, “Making the elements in the Universe,” Europhys. News 44, 23 (2013).
P. Armbruster and G. Münzenberg, “An experimental paradigm opening the world of superheavy elements,” Eur. Phys. J. H 37, 237 (2012).
S. Hofmann, “The discovery of elements 107 to 112,” EPJ Web Conf. 131, 06001 (2016).
K. Morita et al., “Experiment on the synthesis of element 113 in the reaction 209Bi(70Zn,n)278113,” J. Phys. Soc. Jpn. 73, 2593 (2004).
Yu. Ts. Oganessian and V. K. Utyonkov, “Super-heavy element research,” Rep. Prog. Phys. 78, 036301 (2015).
Yu. Ts. Oganessian and K. P. Rykaczewski, “A beachhead on the island of stability,” Phys. Today 68, 32 (2015).
S. Hofmann et al., “Review of even element super-heavy nuclei and search for element 120,” Eur. Phys. J. A 52, 180 (2016).
Yu. Ts. Oganessian et al., “Measurements of cross sections for the fusion-evaporation reactions 244Pu (48CA,xn)292−x114 and 245Cm(48Ca,xn)293−x116,” Phys. Rev. C 69, 054607 (2004).
Joint Institute for Nuclear Research, “Livermorium-288 has been synthesized for the first time in the world at the JINR Laboratory of Nuclear Reactions” 23 October 2023; https://www.jinr.ru/posts/v-lyar-oiyai-vpervye-v-mire-sintezirovan-livermorij-288/.
About the Author
Sophia Heinz is an experimental nuclear physicist. She is a staff scientist at GSI Helmholtz Centre for Heavy Ion Research, an associate professor at Justus Liebig University Giessen, and a lecturer at Philipps University of Marburg, all in Germany. Her research focus is fusion and deep-inelastic transfer reactions in heavy-ion collisions with stable and radioactive projectile beams, aiming at the synthesis and study of new heavy and superheavy nuclides.





Коментарі
Дописати коментар